Learn how to standardize quality assurance procedures in manufacturing to improve overall quality and reduce errors.
It takes implementing just one wrong procedure for a product to end up defective and nowhere near ready for customer delivery. That’s why it’s important to standardize quality assurance (QA) procedures to ensure conformity on the shop floor and prevent product malfunctions.
But what is quality assurance? According to TechTarget, it is a process used to determine whether a product or service meets necessary requirements, in manufacturing specifically these are required standards for distribution. In a nutshell, QA procedures ensure customers receive quality products that are free of defects.
Learn how to standardize quality assurance procedures in manufacturing by exploring the following content:
- What are quality assurance procedures?
- How to standardize quality assurance procedures
- Benefits of implementing QA procedures in manufacturing
What are quality assurance procedures?
QA procedures are a systematic process of establishing and maintaining set requirements for manufacturing reliable products and services. These procedures should be standardized by setting up a quality assurance system for workers to access. There, they can see how to complete certain tasks to avoid errors on the production floor.
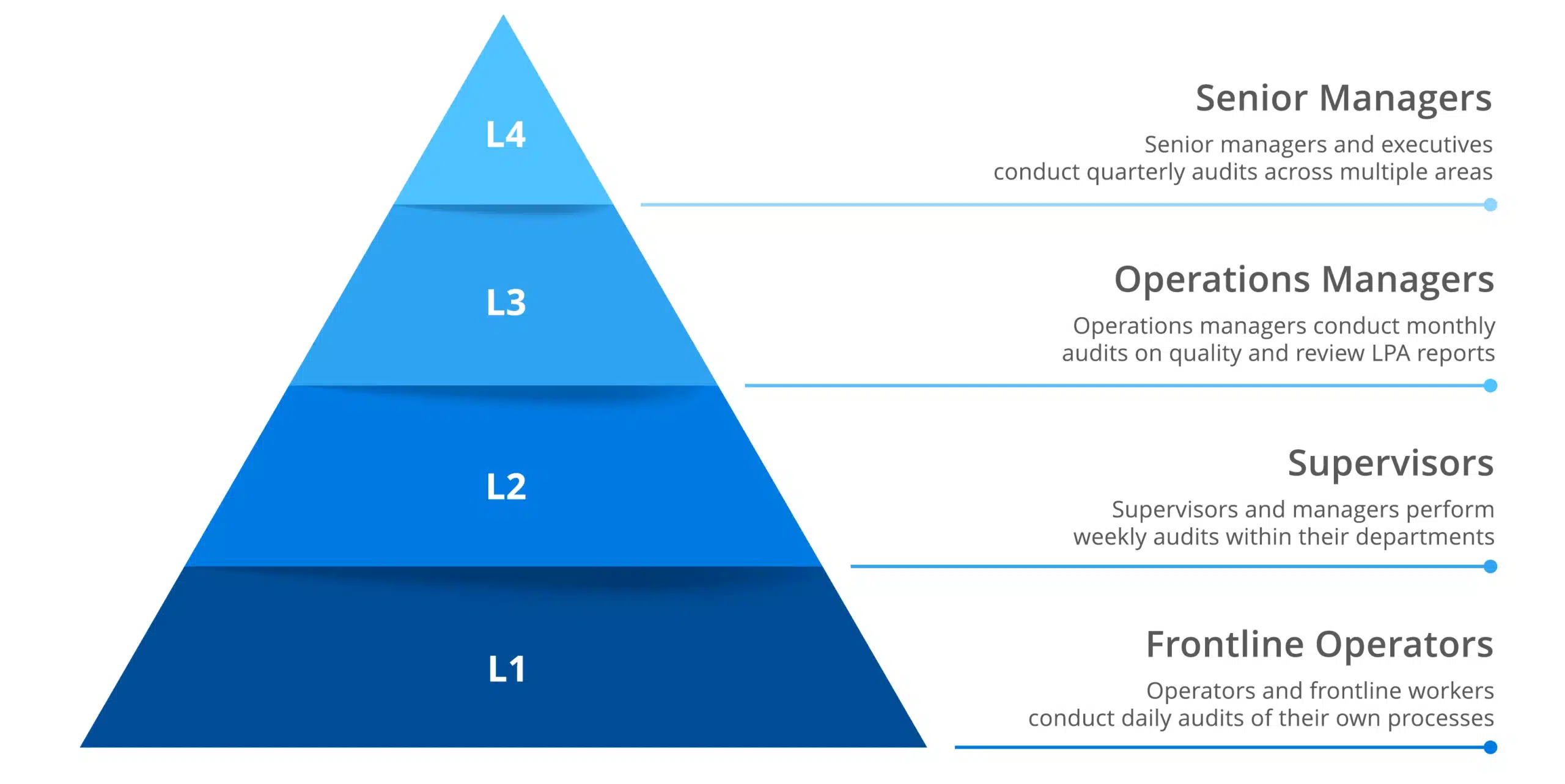
Quality assurance methods can be categorized into three types, which we explain in the table below.
| Type of QA method | Description | Example |
|---|---|---|
| Failure testing | This is the process of testing a product to see if it can withstand stress. The purpose is to identify any deficiencies. | Manufacturers may place a product under heat, pressure, or vibration to test outcomes. |
| Statistical process control (SPC) | SPC is an industry-standard practice for measuring and controlling product quality during the production process. Data is collected by measuring process inputs (dependent variables) in real time. This data is then visualized in SPC charts with predetermined control limits based on how a type of product is expected to perform. | A manufacturing line would apply statistical and analytical tools to monitor input variables and look for excesses or waste. |
| Total quality management (TQM) | TQM is the idea that every employee, from assembly line workers to leadership, is committed to improving processes, products, and services. | TQM may be implemented to raise overall productivity and make a manufacturer more competitive. |
How to standardize quality assurance procedures
Quality assurance procedures help manufacturers develop products and services that meet customers’ needs and expectations. If implemented successfully, QA can catch any defects before they arise and substantially increase product quality.
It’s also vital to implement a quality assurance system to improve efficiency. Developing a unified system makes it easier to incrementally improve your production processes, and it’s essential for standardizing your quality assurance procedures.
Read on about the seven steps for successful QA implementation:
Step 1: Define Organizational Goals
Successful manufacturing QA begins by identifying how workers’ jobs are tied to an organization’s goals. It’s crucial for workers to know their company’s mission and how they fit into it. When employees understand how their individual goals relate to the organization’s goals, it can boost worker confidence — and in turn, production efficiency.
Step 2: Pinpoint Necessary Success Factors
It’s important to list the factors that make your quality assurance process successful. For instance, factors can include production processes, technical or customer support, and other things that make your organization great. Creating a list of major factors that contribute to company achievements will make it easier to update and manage those factors later on.
Step 3: Identify Your Customer Base
It’s vital to define your client case. If you know your customer, you’re more likely to create products and services that they would value.
Step 4: Gather Customer Feedback
Once you’ve established your customer base, it’s vital to incorporate what they think about your products and services. Frequent customer feedback can keep your quality assurance on track since it helps you identify and resolve product issues before they become critical problems. Reports can be gathered though surveys, email, phone, focus groups, or other methods. The goal is to achieve continuous feedback regardless of which methods you choose.
Step 5: Strive for Continuous Improvement
Quality assurance goes hand in hand with continuous improvement. The information you’ve gathered from customer surveys or other methods can now be used to implement any needed changes to your quality assurance process.
Continuous improvement can also be in the form of customer service training, changes to production processes, improvements to products or services, or anything that adds value to your organization.
Whatever you do, it’s crucial to study customer comments and use them to enhance operational procedures to ensure you’re delivering products that bring value and sell.
Step 6: Find QA Management Software
Once you’ve established the above steps, it’s time to start thinking about which quality QA software, or system, will help you better implement QA procedures. Picking the right software will aid with maintaining and improving production processes.
Step 7: Assess Results
Lastly, it’s important to measure your results. Your main goal is to ensure that your business meets the needs of each customer. Create measurable objectives for employees so that everyone knows what needs to be accomplished in a timely manner. If goals aren’t met, make sure workers are clear about what actions need to be taken to meet client satisfaction.
Take note: If your manufacturing firm does not reach its goals, it is hard to show a positive ROI to stakeholders. That’s why taking corrective action to meet company targets is more imperative than ever before.
Benefits of Implementing QA Procedures in Manufacturing
Quality assurance in manufacturing can offer a wide variety of benefits if management makes it a priority.
Some benefits of standardizing QA procedures include:
Cost-effectiveness: When done right, QA can prevent quality product issues before hitting the market. For instance, manufacturers won’t have to worry about scrapped parts, product returns, or other expenses due to poor-quality goods.
Greater workplace efficiency: Manufacturers will be able to better allot resources like time, money, and warehouse space if fewer product deficiencies exist. It boils down to this: it takes fewer resources to develop quality items if processes are in place to support the feat of QA procedures.
Enhanced Customer satisfaction: Customers will almost surely receive quality products in a timely manner if workers employ quality assurance techniques. If fewer product malfunctions exist, customers are more likely to keep coming back for more. In the end, it’s a win-win situation for both businesses and clients alike.
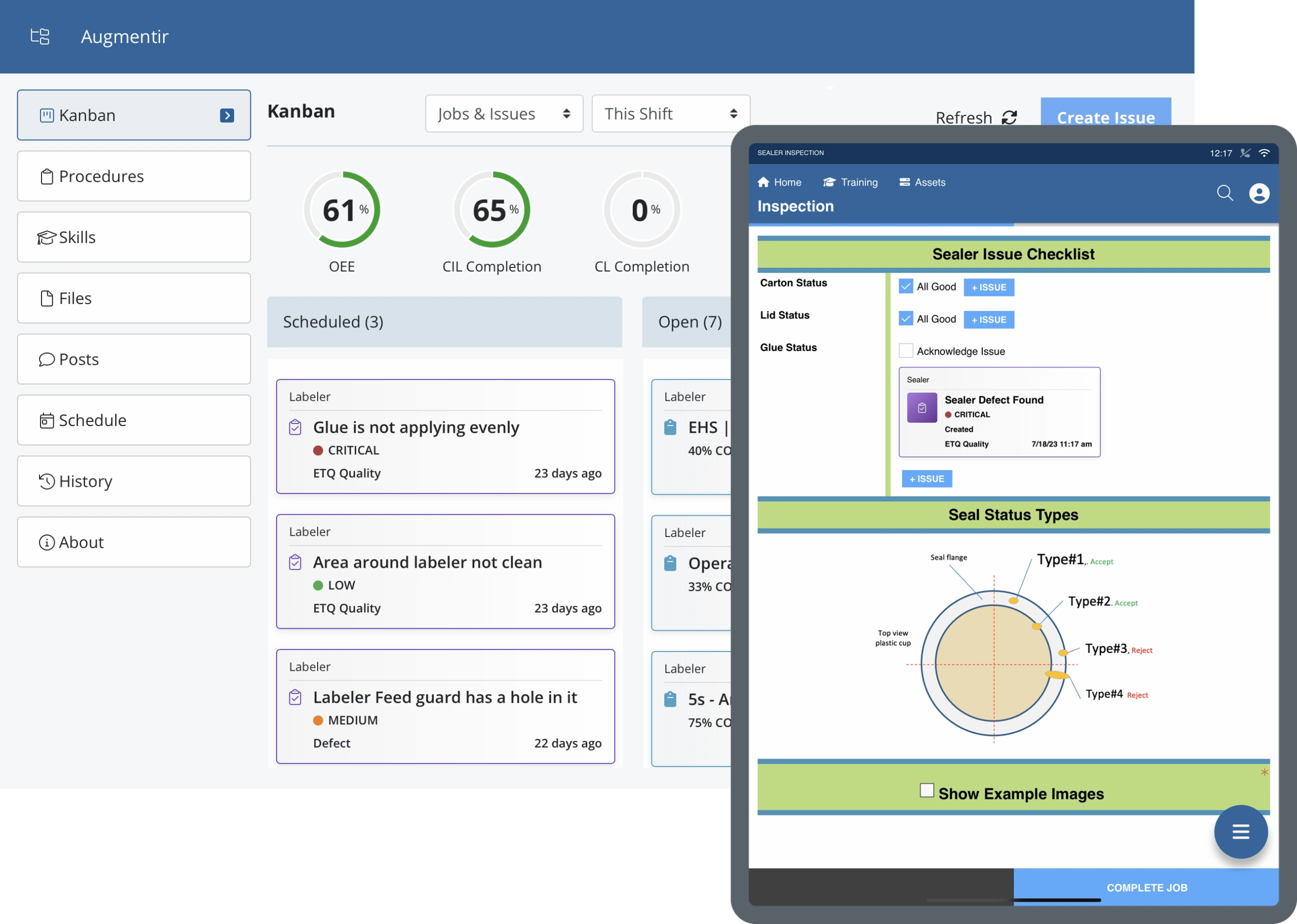
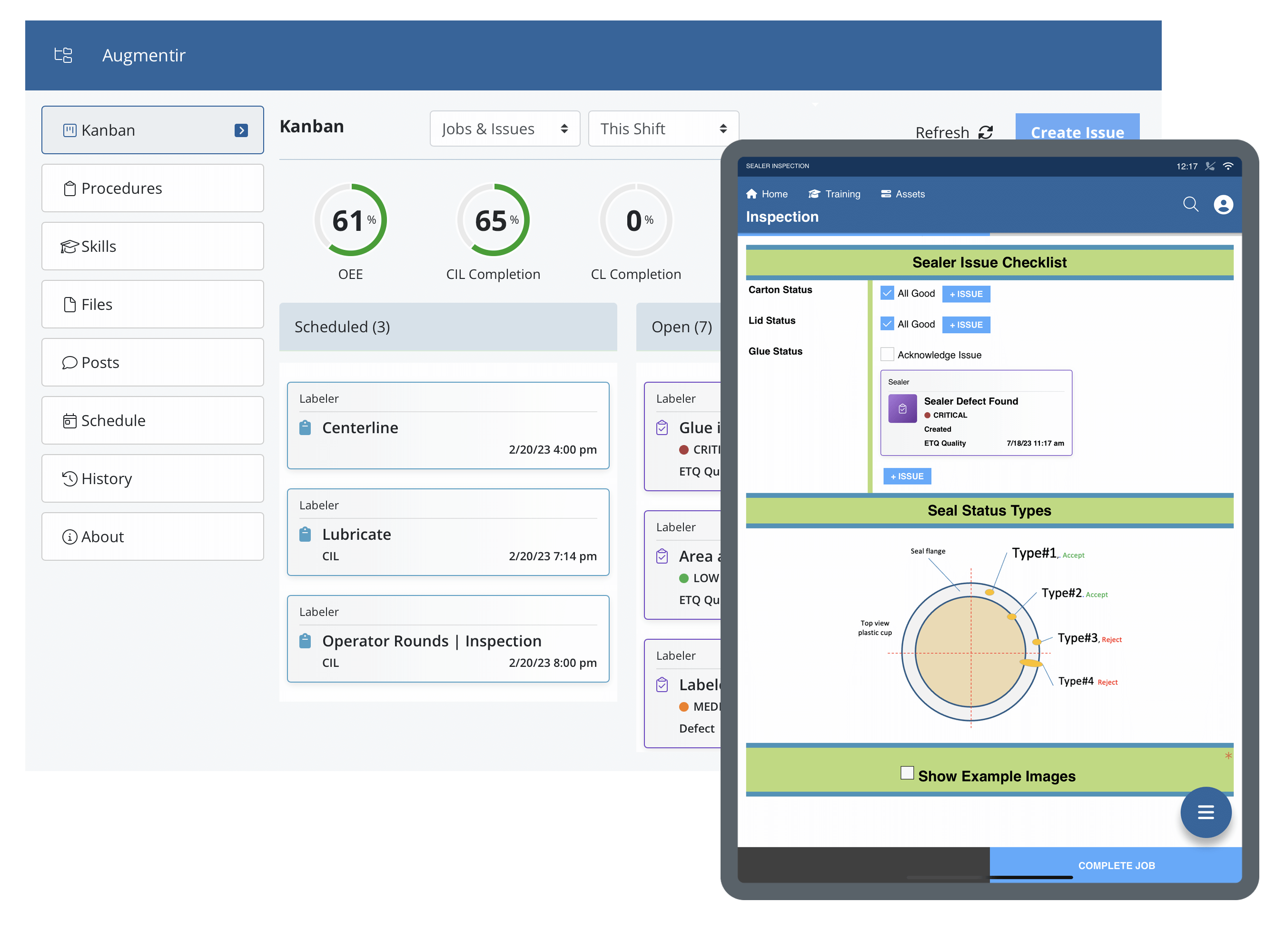
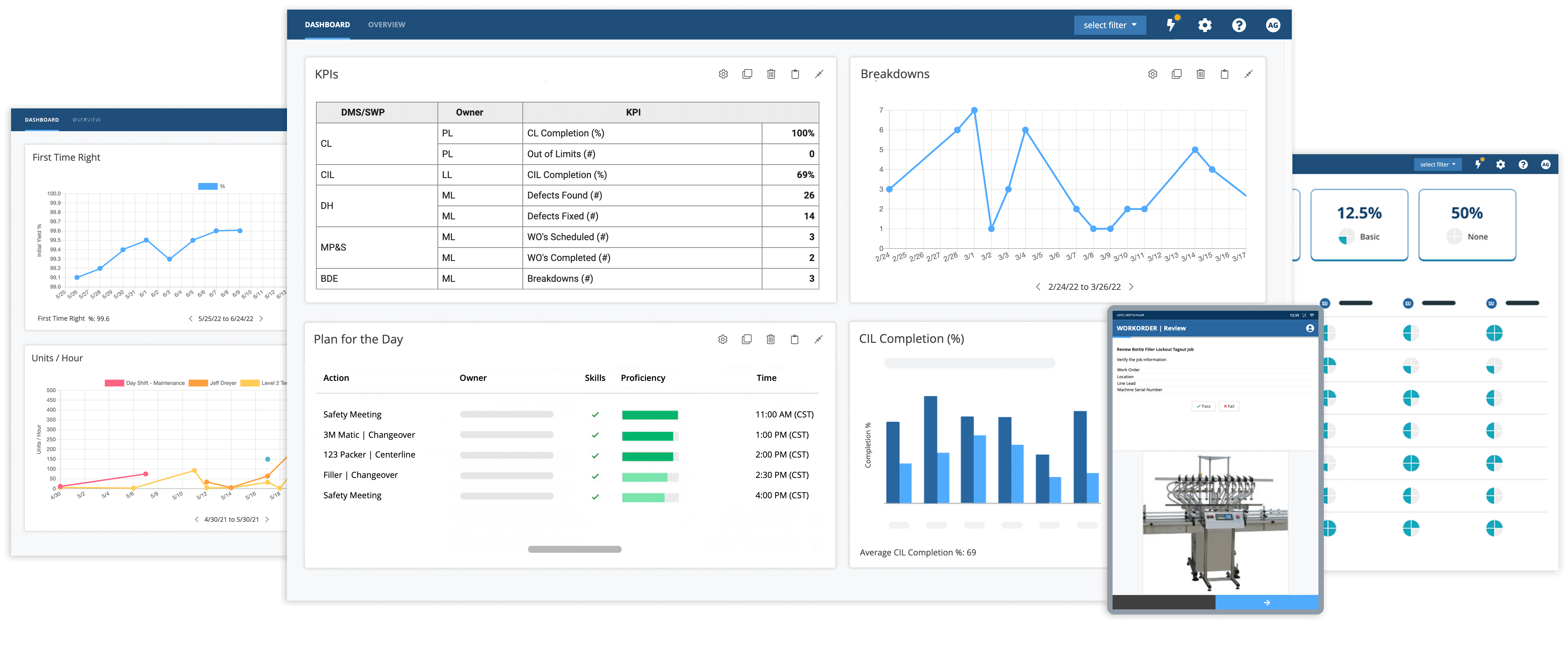
Industrial companies use Augmentir’s breakthrough system to standardize and optimize quality assurance procedures in manufacturing. With Augmentir, you will experience fewer errors and reduced product defect rates with our connected worker solution. Learn more about our quality use cases.
Contact us for a live demo to start optimizing your frontline operations today!